Introduction
Chronic wounds, particularly in diabetic patients, have a significant impact on the patient’s quality of life and those close to them. Various approaches have been explored to address the debilitating consequences of chronic wounds in diabetes, and one emerging field of science, known as catalytic nanomedicine, holds promise in this regard. Catalytic nanomedicine focuses on the design, synthesis, characterization, and application of bionanocatalysts in the medical field. These bionanocatalysts are nanostructures composed of mixed oxides, possessing catalytic properties that selectively disrupt nucleic acids through organic functionalization on their surface, resembling cellular ligands. They are designed to be cytotoxic to pathogens or damaged cells while sparing healthy cells in the body, making them suitable for disinfection and cancer therapy. In the context of chronic wounds, these nanostructures have demonstrated potential as tissue repair agents.
The current work explores the mechanisms of action of bionanocatalysts, particularly their application in chronic wounds for tissue regeneration. Additionally, it discusses the significance of bionanocatalysts in disinfection, their ability to target a wide range of microorganisms, and their unique properties that enable them to act even in the presence of protective biofilms. To put the above into context, a brief description of diabetic pathology is given, contrasting the physiological processes of glycemic regulation with the pathophysiological processes responsible for this disease that can lead to the chronic injury known as diabetic foot. This phenomenon is briefly presented in its pathological components in such a way that the impact of bionanocatalysts in each of the stages of the tissue regeneration process in patients with this disease is evidenced. Furthermore, the article presents a success story of a patient with a diabetic chronic wound, highlighting the effectiveness of bio- nanocatalysts in promoting wound healing. Finally, the article discusses the molecular interpretation of chronic wound repair by bionanocatalysts and provides insights into potential future applications and the need for further research in this field.
By exploring the field of catalytic nanomedicine and its potential applications in chronic wounds, this article aims to shed light on a promising approach that could significantly improve the treatment and healing outcomes for patients suffering from this debilitating condition.
Physiology and glucose regulation
Insulin
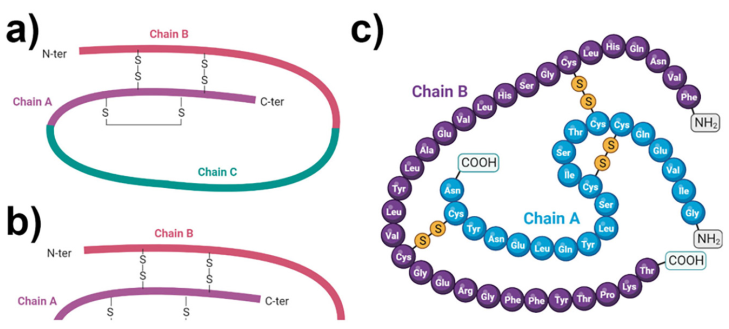
Insulin is a protein hormone made up of two polypeptide chains called the A and B chains that are linked together by disulfide bonds (Petersen and Shulman, 2018) (Figure 1). The A chain has 21 amino acids while the B chain has 30 amino acids (De Meyts, 2004). Two disulfide links connect the two chains, while an extra disulfide bond inside the A chain helps maintain the overall structure of insulin. Insulin folds into a three-dimensional form that allows it to connect to insulin receptors on target cells. Insulin is produced in the pancreatic beta cells as an inactive precursor known as preproinsulin (M. Liu et al., 2014). Preproinsulin is modified post-translationally in the endoplasmic reticulum and the Golgi apparatus. The signal peptide is cleaved, resulting in the conversion of preproinsulin to proinsulin. Proinsulin is made up of three chains: A, B, and a linking peptide termed the C peptide. The C peptide is then cleaved, resulting in mature insulin production. Mature insulin is bundled into secretory vesicles and kept until it is activated by glucose and released into the circulation.
Figure 1
Insulin. Structure of (a) proinsulin and (b, c) insulin. (c) Amino acid chain composition in insulin (c) showing the interaction of chains A and B with sulfhydryl bridges. Regulation of glucose transport.
Source: Author’s elaboration (made in BioRender.com).
Insulin is essential for maintaining glucose homeostasis in the body (Tokarz, MacDonald, and Klip, 2018). Insulin is released into the bloodstream when blood glucose levels rise, such as after a meal. It regulates glucose absorption and is used in a variety of tissues, including the liver, muscle, and adipose tissue. Insulin stimulates glucose absorption by facilitating its transport across cell membranes, especially in skeletal muscle and adipose tissue. It also increases glycogen production in the liver and muscles, resulting in glucose storage.
Insulin regulates lipid and protein metabolism in addition to glucose metabolism (Saltiel and Kahn, 2001a). It slows the breakdown of stored fats (lipolysis) while increasing fat production in adipose tissue. Insulin also increases protein synthesis and prevents protein breakdown, facilitating muscle tissue development and maintenance. Insulin has impacts that extend beyond glucose metabolism. It has an impact on gene expression, cellular proliferation, and differentiation in a variety of tissues. It also regulates hunger and satiety, as well as influencing the cardiovascular system’s function.
Under normal conditions, ATP concentrations keep the channels open and contribute substantially to maintaining the resting membrane potential of β-cells. When the glucose level rises, it enters the β-cells by a specialized transport process mediated by GLUT2 glucose transporters (facilitated diffusion). Glucose is metabolized inside the cell to glucose-6-P by glucokinase, increasing the intracellular ATP level. The increase in ATP causes the closure of the K+ channel and the outflow of this ion, so that the β-cell undergoes a depolarization that compensatorily activates the Ca2+ channels, resulting in Ca2+ entry and initiation of Ca2+ -dependent processes, which ultimately favor the release of insulin. Glucose at elevated concentrations sensitizes the cell, so that it facilitates increased insulin secretion, triggered by other stimuli (Saltiel and Kahn, 2001b; Ebina et al., 1985) (Figure 2).
Figure 2
Metabolism of insulin and molecular pathways involved on its release from beta-cells.
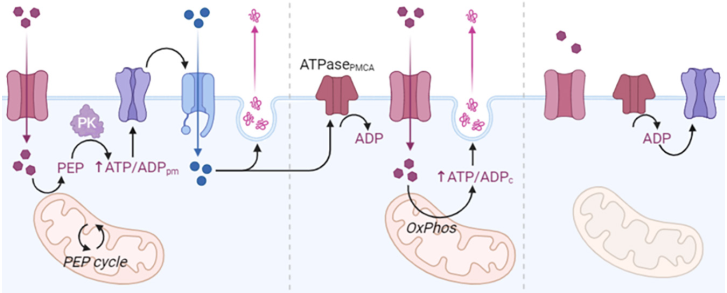
Source: Author’s elaboration (made in BioRender.com).
Insulin activates the MAPKs pathway (MAPKs are a family of serine/threonine kinases activated by growth and stress factors) (Lawrence et al., 2008). These proteins play a key role in intracellular signal transduction, allowing the cell to integrate different extracellular stimuli through two mechanisms: Insulin receptor activation promoting SHC protein association, which are SHC1 transforming proteins, found in humans and encoded by the SHC1 gene. SHCs have been found to be important in the regulation of apoptosis and drug resistance in mammalian cells (Ravichandran, 2001).
The α-subunits contain insulin-binding regions α1IR and α2IR in addition to a cysteine-rich (Cys) region. Whereas the β-subunits contain an extracellular, a transmembrane and an intracellular portion (Molecular basis of insulin action, 2007). In its intracellular portion is located the kinase and tyrosine catalytic domain with an ATP binding site and tyrosine phosphorylation sites that are located in the juxtamembrane regions, with carboxyl-terminal activation (Zick, 2005). Under non-stimulus conditions, the α-subunits regulate the β-subunits, inhibiting the autophosphorylation capacity of the receptor. The mechanism of autophosphorylation is carried out by cis- and trans- autophosphorylation processes (Mancusi et al., 2020): CIS-Phosphotransferase activity. Residues are phosphorylated by their own β-subunit (in cis). TRANS-Kinase activity. Residues are phosphorylated by the opposite β-subunit (in trans). Recent studies indicate that at least 7 Tyr phosphorylation sites in the IR and Tyr kinase enzymatic activity are required for proper receptor function (Youngren, 2007).
These bind to the Grb2/SOS complex; SOS and activates Ras, which initiates the activation of the MAPKs cascade (Chen et al., 1996) (Figure 3). GTP-Ras binds and activates Raf-1 which subsequently leads to phosphorylation and activation of MEK and ERK1/2. Alternative pathway independent of SHC, but dependent on insulin receptor substrate (IRS) activation by which insulin is able to activate MAPKs. In this pathway, once IRS is active, it binds to the Grb2/SOS complex and from this point the protein activation sequence is the same as for SHC. Regulates gene expression in insulin-sensitive tissues and mediates the effects for proper receptor function.
Figure 3
Activation of MAPK proteins by insulin action. Insulin activates the MAPK pathway through two mechanisms. In the first, activation of the IR promotes the association of the Shc protein, which binds the Grb2/SOS complex; SOS activates Ras, which initiates the activation of the MAPK cascade.
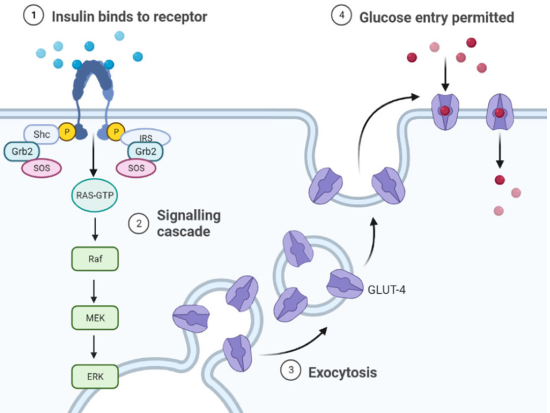
Source: Author’s elaboration (made in BioRender.com).
Diabetes mellitus
Definition
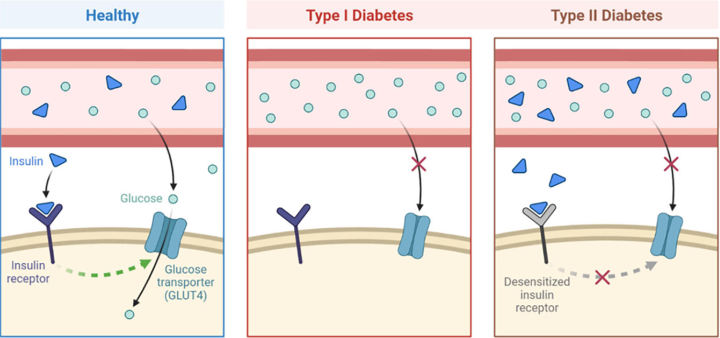
Diabetes mellitus (DM) is a chronic metabolic disorder characterized by the occurrence of a constant state of hyperglycemia in the blood (Moini, 2019). The World Health Organization (WHO) reported a prevalence of 422 million people with diabetes (PAHO, 2022). Since its first description 3000 years ago, our understanding of DM has deepened in terms of pathophysiology and development of treatments for the preservation of a healthy life, even when this disease remains incurable. Two types of DM have now been identified, type 1 (T1DM) and type 2 (T2DM), which are characterized by the absence or reduced secretion of insulin, and damage to insulin receptors, respectively (Petersmann, et al. 2019) (Figure 4).
T1DM, also known as type 1 diabetes mellitus, insulin-dependent diabetes mellitus (IDDM) or juvenile diabetes, accounts for approximately 5-10% of all patients with diabetes (Banday, Sameer and Nissar, 2020). It is an autoimmune disease characterized by apoptosis of pancreatic beta cells by T cells, resulting in insulin deficiency and ultimately hyperglycemia (Knip and Siljander, 2008; Kahaly and Hansen, 2016). Although the etiology of this autoimmune disease is not yet fully understood, both hereditary and environmental factors have been shown to be involved. This pancreatic cell-specific autoimmunity and the disease itself usually develop rapidly in infants and children (juvenile onset) (Bimstein et al., 2019). However, the disease can also develop gradually in adulthood (late onset).
On the other hand, T2DM, often referred to as non-insulin-dependent diabetes mellitus (NIDDM) or adult-onset diabetes in older terms, accounts for 90-95% of all diabetes (Virally et al., 2007). Insulin resistance and cellular dysfunction are the two main insulin-related diseases that define this type of diabetes (DeFronzo, 2004; Muoio and Newgard, 2008; Galicia-García, et al. 2020). Disorders of multiple cellular pathways cause insulin resistance and reduce insulin sensitivity of cells in peripheral tissues, especially muscle, liver, and adipose tissue. Increased insulin secretion to maintain normoglycemia is achieved early in the disease state, when insulin sensitivity is reduced, and cells are hyperfunctioning. Thus, hyperinsulinemia and increased circulating insulin prevent hyperglycemia. However, over time, the increased insulin secretion by the cells cannot fully compensate for the reduced insulin sensitivity. In addition, cell function begins to deteriorate and cell dysfunction eventually leads to insulin deficiency. As a result, normoglycemia cannot be maintained and hyperglycemia occurs.
Pathophysiology of diabetes
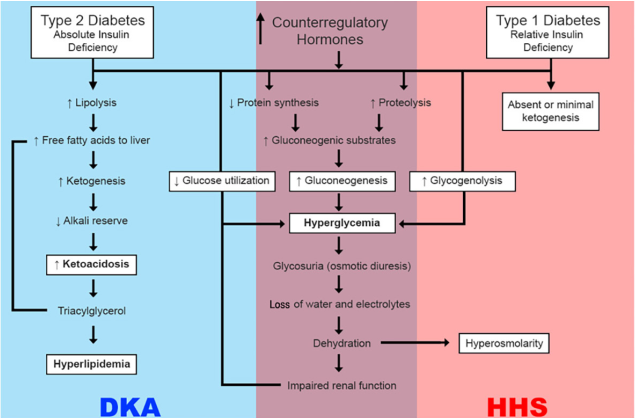
The continued state of insufficient or absent insulin capable of allowing glucose uptake can result in diabetic ketoacidosis (DKA) or hyperosmolar hyperglycemic state (HHS), the two extremes of decompensation of type 1 and type 2 diabetes, respectively (Kitabchi et al., 2009) (Figure 5). DKA is characterized by the biochemical triad of hyperglycemia, ketonemia, and high anion gap metabolic acidosis (Kitabchi and Wall, 1995). HHS, on the other hand, although also characterized by hyperglycemia and absolute or relative insulinopenia, differs clinically in the severity of dehydration, ketosis, and metabolic acidosis (Hassan et al., 2022).
In both scenarios, the inappropriate utilization of glucose for energy production in the cells results in severe impairment of carbohydrate, protein, and lipid metabolism (Kitabchi et al., 2001). The body shifts to a state of increased catabolism with breakdown of glycogen stores, hydrolysis of triglycerides from adipose tissues, and mobilization of amino acids from muscle. Triglycerides and amino acids released to peripheral tissues become substrates for the production of glucose and ketone bodies in the liver.
The following deficiencies underlie DKA and HHS: 1) decreased insulin secretion (DKA) and impaired insulin CHC action, resulting in reduced net circulating potency of insulin (Chupin, Charbonnel and Chupin, 1981; Kipnis, 1968; Kitabchi, 1976); 2) increased levels of inverse regulatory hormones such as glucagon (Kitabchi et al. 1979; Müller, Faloona and Unger 1973), catecholamines (Kitabchi et al., 1979; Christensen, 1974), cortisol (Kitabchi et al., 1979), and growth hormone (Müller, Faloona and Unger, 1973; Christensen, 1974) (Figure 1). Other signs of DKA include increased lipolysis, ketogenesis, increased glycogenesis, and impaired glycolysis (Kitabchi and Nyenwe, 2006).
Pathophysiology of the diabetic foot
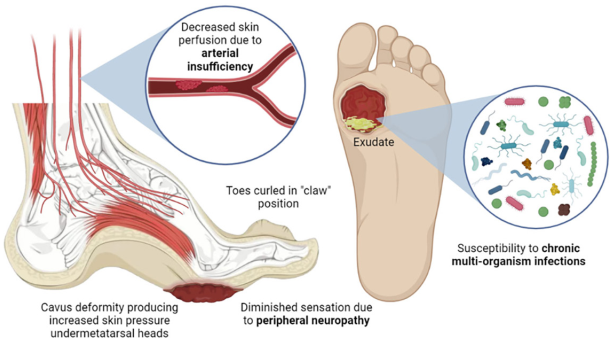
Taken together, the molecular-level effects of the pathological increase in blood glucose concentration (hyperglycemia) result in the triad associated with the diabetic foot: peripheral neuropathy, arterial insufficiency, and infection. In the present section we will describe in a simple way the molecular processes that lead to each of these points.
Peripheral neuropathy
Peripheral neurons of the lower extremities being the longest cells in the body, their function requires vascular supply, mitochondrial action and adequate glucose and lipid metabolism, all of which are altered in DM. Under conditions of hyperglycemia, proteins, lipids, and nucleic acids can undergo irreversible enzymatic reactions, forming advanced glycation end products (AGEs). When such metabolic products are formed throughout the peripheral nervous system, including nerve axons, neural microvasculature, Schwann cells, and the extracellular matrix, significant structural and functional changes are induced (Wada and Yagihashi, 2005). In addition, the interaction of AGEs with their receptors activates intracellular signaling pathways that cause downstream inflammation, oxidative stress, and nuclear DNA degradation, ultimately resulting in vascular dysfunction and nerve conduction deficits (Vincent et al., 2007).
Atrophy of the lumbrical and interosseous muscles results in functional anatomical changes, such as hammer-toe formation and the development of high-pressure zones on the plantar surface of the metatarsal heads. Repeated gait injuries, along with decreased sensitivity and proprioception, can easily cause skin damage, atrophy, and displacement of the protective fat pad of the sole, inadequate skin protection, and improper footwear can cause ulcers (Bandyk, 2018).
Arterial insufficiency
In addition to nerve damage, changes in glucose metabolism and hyperglycemia lead to endothelial damage, hyperlipidemia, increased viscosity, and platelet activity (Youn and Lee, 2019) and, over time, the development of atherosclerosis, which occurs due to the accumulation of fatty deposits and cholesterol in the arterial walls (Libby, 2021). In this context, circulation, mainly of the posterior and anterior tibial arteries, is reduced, so that blood moves more slowly. With the development of this occlusive arterial disease, the reduced perfusion to the foot results in reduced biodistribution of nutrients and growth factors necessary for the maintenance of skin integrity (Lepäntalo, Fiengo and Biancari, 2012). These alterations include impaired sweating, dry and cracked skin, collagen production reduction, weakened skin barrier and reduced production and repair of new blood vessels (Morton and Phillips, 2016). Furthermore, since oxygen is crucial for cellular metabolism and the production of energy needed for wound healing processes, an inadequate oxygen supply, known as tissue hypoxia, further delays the healing process in chronic wounds (Hiatt et al., 2015). This contributes to the risk factors associated with peripheral neuropathy for the development of limb ulcers.
Infection
Poor glycemic control leads to immune dysfunction with altered leukocyte activity (Geerlings and Hoepelman, 1999; Frydrych et al., 2018; Golden and Simmons, 2021). Reduced white blood cell function facilitates the rapid development of invasive tissue infections (Jenks et al., 2020). Indeed, skin damage in patients with poor blood perfusion can lead to rapid penetration of bacteria deep into the fascia. Polymicrobial infections, with families of microorganisms such as staphylococci, streptococci, enterococci, E. coli, and others, are common in diabetic foot wounds (Wu, Cheng and Cheng, 2019; Luo et al., 2022). The presence of bacteria triggers an inflammatory response that can overload the local immune system and impede the formation of new tissue (Hirano, 2021). Prolonged inflammation not only inhibits wound healing but can also damage surrounding healthy tissue and prevent the formation of new blood vessels (Li et al., 2021).
Particular danger exists when multi-resistant strains to antibiotics are present. Bacterial biofilms, which are common in chronic wound infections, are more resistant to antibiotics (Versey et al., 2021). These biofilms form a protective barrier that allows bacteria to evade the immune system and makes them less susceptible to antimicrobial agents. The reduced efficacy of antibiotics further complicates the treatment of infected chronic wounds (Yin et al., 2019).
In addition, infection in chronic wounds increases the risk of complications. Bacterial overgrowth can lead to breakdown of surrounding tissue, deteriorating wound margins, and causing wound enlargement (Hua et al., 2023). In severe cases, infection can spread to deeper tissues, such as muscle or bone, resulting in osteomyelitis or cellulitis (Forsberg et al., 2011). In addition, infected wounds are more prone to excessive exudate (fluid discharge), bad odor and increased pain (Cutting, 2003). In severe cases, a chronic wound infection can cause systemic effects. Bacteria and their by-products can enter the bloodstream and cause a systemic infection (sepsis). Sepsis is a life-threatening condition that can cause organ dysfunction and failure if not treated promptly (Youn and Lee, 2019).
Chronicity of the wound
Although the body normally has tissue repair systems for wound repair, following the stages of (i) hemostasis, (ii) inflammation, (iii) proliferation and (iv) remodeling, the triad of the diabetic foot prevents proper wound healing, making the wound chronic. Generally speaking, these chronic wounds are characterized by excessive levels of proinflammatory cytokines, proteases, reactive oxygen species, senescent cells, bioburden, and a deficiency of functional stem cells (Frykberg and Banks, 2015).
The main problem associated with infections in chronic wounds of the diabetic patient is the appearance of biofilms. These polymeric structures form when bacteria attach to a surface and use quorum-sensing molecules to induce changes in gene expression, resulting in a barrier composed of 85% exopolymers (polysaccharides, proteins, nucleic acids) and 15% bacteria (Goldberg and Diegelmann, 2020).
Biofilms have been observed to be more recalcitrant than bacteria to the host immune response, making them a greater challenge for the treatment of chronic wounds than bacteria alone. This is because leukocytes, already affected by infection per se, find it difficult to penetrate and maneuver through the biofilm, thereby reducing their ability to eliminate infection by producing reactive oxygen species. Furthermore, when incorporated into biofilms, bacteria create a microenvironment in which they may have reduced metabolic activity, rendering them less susceptible to antimicrobial agents that attack metabolically active cells (Peterson, 2005). Finally, biofilms stimulate a chronic inflammatory response that perpetuates the chronic wound senescence cycle without allowing tissue regeneration (Wolcott, Rhoads and Dowd, 2008).
Taken together, the appearance of biofilms in the wound, as well as underlying causes such as arterial insufficiency that impedes the transport of tissue repair agents and peripheral neuropathy that inhibits ulcer detection, result in a chronic wound response that impedes wound healing, as summarized in Figure 6 below.
Hyperglycemia, among other factors, derive in the triad: peripheral neuropathy, arterial insufficiency, and infection. Anatomically functional deformations of the feet derived from peripheral neuropathy create high-pressure zones susceptible to ulcer formation. Given the diminished sensation in the zone, the ulcer grows and persists due to the decreased skin perfusion associated with arterial insufficiency that, otherwise, would provide the necessary machinery for wound repair. These conditions facilitate the development of infections in the area which, in turn, impede tissue regeneration.
Catalytic nanomedicine in chronic wounds
Being nowadays this condition of such a serious impact on the quality of life of the patient and his close people, different approaches have tried to compensate the disabling consequences derived from chronic wounds in diabetic patients. In this context, the new branch of science called catalytic nanomedicine becomes relevant.
Catalytic nanomedicine, as described by López-Goerne et al. (2022), is the branch of nanotechnology focused on the design, synthesis, characterization, and application of bionanocatalysts in the medical field (López-Goerne et al., 2022). These nanostructures are composed of mixed oxides that exhibit catalytic properties capable of selectively destabilizing nucleic acids due to the organic functionalization of their surface that simulates cellular ligands (López-Goerne, 2013 y 2011). These nanoparticles are designed to be selectively cytotoxic towards pathogens or damaged cells, without affecting healthy cells of the organism in the process: this makes them particularly suitable for disinfection and cancer therapy. The following sections will describe the mechanisms of action of these nanostructures, particularly around their application as tissue repair agents in chronic wounds. A more detailed description of Catalytic nanomedicine and bionanocatalysts can be found elsewhere (López-Goerne et al., 2022).
Bionanocatalyst-mediated disinfection
In the field of disinfection, the bacteriostatic properties of bionanocatalysts based on nanostructured titanosilicate on which copper nanoparticles (CuNPs) are deposited as a coating have been demonstrated (Jiménez et al., 2022). CuNPs themselves exert a size-dependent antibacterial activity, being able to internalize the cell by disrupting the bacterial protective barrier and degrading the plasmid-like genetic material of Gram-positive and Gram-negative microorganisms (Chatterjee, Chakraborty and Basu, 2014; Ramyadevi et al., 2012; Crisan, Teodora and Lucian, 2021; Chand Mali et al., 2023). When used as coating for bionanocatalysts, they are optimized given the intrinsic DNA degradation properties of bionanocatalysts.
Such degradation follows what is known as “complete combustion by three-way converter” (Hayes et al. 2004). The bionanocatalyst catalyzes, by breaking C-C and C-N bonds, the oxidation of carbon monoxide to carbon dioxide, the reduction of nitric oxides to nitrogen and molecular oxygen, and the combustion of carbons to carbon dioxide and water (López-Goerne et al., 2022). This phenomenon is used to destabilize organic compounds and macromolecules (such as DNA), releasing as residues normal organic molecules in the organism: CO2, N2, O2 and H2O (López et al., 2018).
The effect on genetic material results in genotoxicity that inhibits cell division, while ROS are produced that damage the bacterial cytoskeleton and fragment the cell (Liu, Xu, and Slaveykova, 2023). Furthermore, in eukaryotic microorganisms, bionanocatalysts show intrinsic chemotaxis by mitochondria, the cellular energy factory (Yakes and Van Houten, 1997). Destabilization of mitochondrial genetic material causes depletion in ATP production levels and autolysis of the organelle (Cline, 2012). The generation of ROS and the lack of energy lead the cell to enter a state of stress that results in programmed death by apoptosis.
It is important to emphasize that, due to the intrinsic mechanism of action of bionanocatalysts, the microbicidal effect is independent of the sequence of the genetic material, unlike other sequence-specific compounds. This factor gives bionanocatalysts the ability to inhibit the action of a wide range of prokaryotic and even eukaryotic microorganisms, such as Candida albicans (López et al. 2015). The bactericidal effect is therefore directly conditioned by the protective capacity of the microbial cell wall (Christaki, Marcou and Tofarides, 2020). Once inside, the agent is cytotoxic regardless of the composition of the genetic material, whether in the form of nucleoid or nucleus (the bionanocatalyst, due to its size, penetrates through the nuclear pores). The above mechanisms are shown in Figure 7.
Figure 7
Mechanisms of action for disinfectant bionanocatalysts.
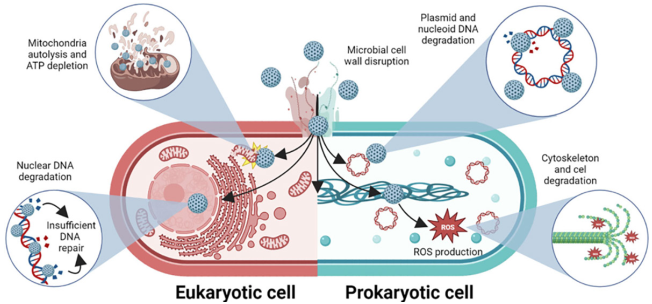
Source: Author’s elaboration (made in BioRender.com).
The nanostructures disrupt microbial cell wall (in Gram-positive, Gram-negative, and yeast microorganisms), and internalize to destabilize several internal structures, such as DNA (in the nucleus or nucleoid, and in the shape of plasmids), mitochondria (in yeasts), and the cytoskeleton (through the production of ROS).
Bionanocatalyst-mediated tissue regeneration in diabetic wounds
The disinfectant capacity of bionanocatalysts is of utmost importance for tissue regeneration in diabetic chronic wounds (Jones, Foster and Longaker, 2018). As described throughout this work, the reduced microcirculation and pathogenesis of diabetic wounds due to hyperglycemia results in altered immune system, decreased nutrient distribution, impaired sweating, dry and cracked skin, and foot abnormalities. These factors together facilitate the formation of infections in exposed tissues, which can easily be aggravated by diabetic neuropathy that inhibits local sensation. Although reduced, the wound response remains present in diabetic patients, so that, with proper care, tissue regeneration can take place (Burgess et al., 2021). However, the formation of polymicrobial biofilms stimulating the chronic inflammatory response associated with diabetic ulcers inhibits the action of macrophages and neutrophils and promotes the preservation of the infection, impeding the action of the regeneration mechanisms (Omar et al., 2017). This is the line of action of bionanocatalysts for tissue regeneration (Figure 8). Unlike other selective disinfectant compounds, bionanocatalysts, due to their mechanism of action focused on genetic material in general (regardless of the nucleotide sequence), are capable of inhibiting the growth of a wide range of microorganisms, both prokaryotic and eukaryotic. In addition, bionanocatalysts have shown the ability to act even in the presence of biofilms that protect microbial aggregates from disinfecting agents (López-Goerne et al., 2022).
Figure 8
Mechanisms of action for tissue-regeneration bionanocatalysts.
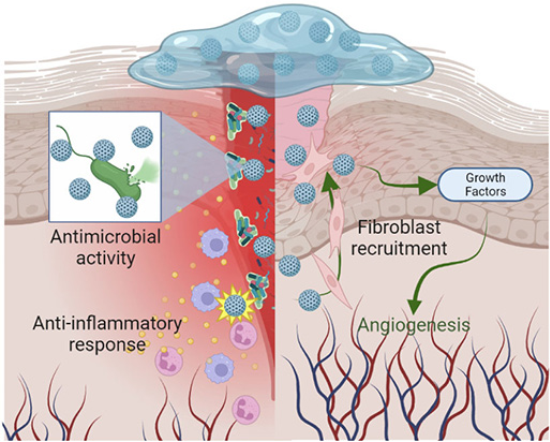
Source: Author’s elaboration (made in BioRender.com).
Their application in nanostructured hydrogels allows the controlled release of the bionanocatalysts homogeneously over the entire surface area of the wound (López-Goerne et al., 2022). Their ultrananoparticulate size (< 15 nm) allows them to pass through the intricate polymeric matrix and reach the microorganisms (Peulen and Wilkinson, 2011), where they carry out the mechanisms of action described in the previous section.
In addition, the nanostructured hydrogel itself acts as a wound protector by providing the temperature and moisture conditions necessary for wound healing by (i) increasing the flow of oxygen and nutrients through angiogenesis, (ii) acidifying the area to create a bacteriostatic environment, (iii) creating a physical barrier that reduces the risk of contamination, (iv) facilitating fibroblast migration, and (v) controlling exudate without damaging the perilesional tissue (Cacicedo González et al., 2011). This is usually complemented by periodic surgical debridement sessions for the removal of wound remnants.
Overall, as demonstrated by Wolcott and Rhoads (2008) and Wolcott et al. (2010), biofilm removal and periodic infection elimination facilitate wound healing.
Success Story
Case report
HCC, a 61-years-old male patient, with a clinical history of DM2 for more than 20 years before our treatment, presented with severe trauma to the distal phalanx of the first toe of the right extremity, with an open penetrating wound on the plantar aspect (Figure 9a). The wound bed exhibits 70% fibrin tissue, 20% sphacelial tissue, and 10% granulation tissue, with abundant exudate. Irregular borders are observed, with severe hematoma in the middle of the toe. The patient had initially been managed with oral and then intramuscular antibiotics; however, rapid tissue degradation was observed due to the presence of a multibacterial infection with presence of Gram-positive and Gram-negative bacteria (Figure 9b). The ineffectiveness of antibiotic treatment for four weeks resulted in a diagnosis of amputation to prevent further spread of the infection. The patient was referred for consultation to determine alternative treatments. In these conditions, the patient was received in our diabetes clinic.
Figure 9
Case report of patient HCC exhibiting a 4-month chronic wound treated with nanogel impregnated with bionanocatalysts in 4 weeks of treatment.
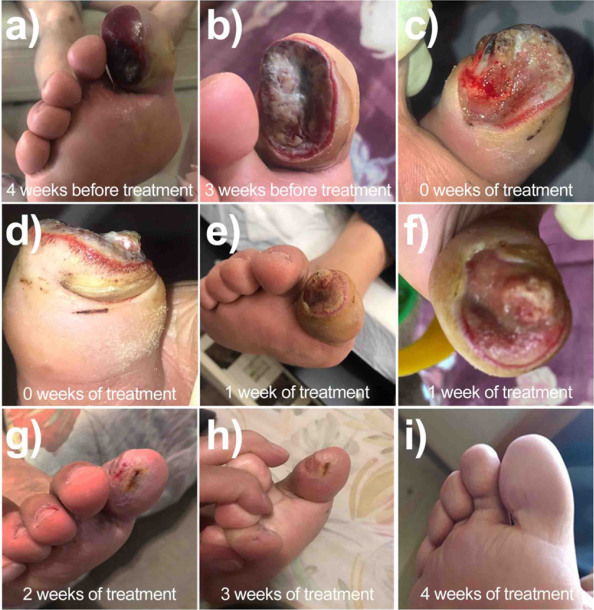
Source: Author’s elaboration.
At the time of our first physical examination, surgical debridement was performed to remove as much infected tissue as possible (Figures 9c and 9d). Subsequently, based on preliminary case studies, a nanogel impregnated with copper titanosilicate bionanocatalysts was applied over the full extent of the wound in conjunction with a bandage. Daily healing was performed for 28 days (weekly debridement and gel application every day), leaving the finger uncovered one day a week with the application by the patient of propolis with bee honey.
From the first week of application, the wound appears clean with a remarkable revascularization and reduction of edema (Figure 9e, 9f). At the two-week mark, a considerable reduction in wound size is observed, with granual tissue formation, with no signs of erythema or edema (Figure 9g). After three weeks, the wound is virtually closed with complete re-epithelialization (Figure 9h). Finally, by the time of discharge, the wound has closed completely and only a slight scar is observed, with no inflammation (Figure 9i).
Molecular interpretation of chronic wound repair by bionanocatalysts
Trauma involves very rapid cell destruction, its contents are released, which are detected by the Langerhans cells of the skin, which will begin to secrete chemo-attractive substances for neutrophils, monocytes and eosinophils (Deckers, Hammad and Hoste, 2018). This will begin to activate the immune system, which will be on alert for the presence of infectious agents that may further complicate the situation (Stoitzner, 2010). Hemostasis begins with the contraction of the smooth muscles of the blood vessels, thanks to the autonomic nervous system, decreasing blood flow to the affected area under normal conditions, the endothelial cells secrete anticoagulant substances, but the rupture of the vessels will cause this balance to be destabilized and the endothelial cells begin to release aggregating substances, such as Von Williebrand factor, a glycoprotein that acts as a bridge between platelets and collagen fibrils (Wang et al., 2019).
The bionanocatalyst acts precisely by eliminating the infectious agents (through the mechanisms described above) and allowing angiogenesis of new blood vessels to the affected area. Unlike other disinfecting agents, bionanocatalysts have an effect on a wide range of pathogenic microorganisms (Jiménez et al., 2022) and, in addition, are able to penetrate the biopolymer network that makes up the biofilm, to selectively and efficiently eliminate the bacteria that make it up and prevent proper tissue regeneration. Thus, the organism can activate its own wound repair mechanisms through the formation of fibrin.
Fibrin formation can occur via two pathways, the extrinsic pathway which is mediated by tissue exposure factor, released at the site of injury and which will act as a cofactor for the activation of factor X, this reaction is catalyzed by factor VII; while another intrinsic pathway occurs by the activation of factors XII and XI, stimulated by platelet aggregation and Von Williebrand factor released by platelets (Weisel and Litvinov, 2017). Then, the two pathways unite to obtain the final product which is fibrin. This filamentous protein binds to the vessel walls to form a mesh that traps the plasma elements, preventing their extravasation and reestablishing hemostasis in the capillaries. This fibrin clot will also play a fundamental role in the beginning of the proliferation phase, acting as a provisional matrix for the migration of fibroblasts; during proliferation the clot will be reabsorbed by macrophages to give rise to the mature matrix for epithelialization. Growth factors and interleukins are then released into the wound by platelets, macrophages, lymphocytes and endothelial cells, so that normal tissue repair can take place (Yamakawa and Hayashida, 2019).
Future perspectives and limitations
The broad-spectrum microbicidal properties of bionanocatalysts and the advantages of using them in nanogels suggest that these nanostructures could be used to treat certain skin diseases caused by chronic infections. In particular, acne, a chronic inflammatory disease caused by the colonization of hair follicles on the face, neck, chest and back by Propionibacterium acnes (Williams, Dellavalle and Garner, 2012), could be treated with antimicrobial bionanocatalysts to eliminate chronic infection and promote tissue regeneration. Similarly, another type of wound that has been treated preclinically are burns. Although preliminary, the results are quite promising. Taken together, bionanocatalysts with regenerative properties could be used in the future for the treatment of a broad spectrum of acute and chronic wounds. To this end, further research is needed on the mechanisms of action of these nanostructures, in particular on the biological processes occurring during the different stages of wound healing and the impact of bionanocatalysts on these phenomena.
It is noteworthy that case series might be biased, limiting their generalizability to broader patient groups. Nonetheless, the information gleaned from the healing processes of the four patients studied in this study allows us to speculate on the efficacy and mechanism of action of bionanocatalysts in chronic wound healing.
Conclusions
Chronic wounds resulting from microcirculation problems, such as diabetic foot, comprise an international epidemic that must be addressed as soon as possible. Although there are many approaches to treat chronic wounds, the approaches are often focused on disinfection or wound protection treatments only, without addressing the underlying molecular processes. In this sense, the present review offered a novel perspective based on the use of bionanocatalysts, which not only act by disinfecting the damaged area, but also promote tissue regeneration by inducing important processes such as the elimination of chronic inflammation and the recruitment of tissue remodeling cells, among other processes. A case report was also presented showing the effectiveness of these nanostructures for the treatment of chronic wounds, with no side effects. It is necessary to continue the research on the application of Catalytic nanomedicine for the treatment of chronic wounds, in order to deepen the areas of opportunity of this branch of knowledge so that it can reach more people and continue with the resolution of this complex problem.
Ethics approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The research protocol was authorized by the Direction of Teaching and Research of the Specialized Center for Diabetic Patients “Dr. Manuel Gonzalez Rivera” under the Ministry of Health of the Federal District, under registration number 101/100/014/13. Informed consent was obtained from all individual participants included in the study. No identifiable information of the patient was included in this work.
References
Banday, Mujeeb Z., Aga S. Sameer and Saniya Nissar. (2020). Pathophysiology of diabetes: an overview. Avicenna Journal of Medicine, 10(04): 174-88. https://doi.org/10.4103/ajm.ajm_53_20.
Mujeeb Z. Banday Aga S. Sameer Saniya Nissar 2020Pathophysiology of diabetes: an overviewAvicenna Journal of Medicine100417418810.4103/ajm.ajm_53_20
Bandyk, Dennis F. (2018). The diabetic foot: pathophysiology, evaluation, and treatment. Seminars in Vascular Surgery, 31(2-4): 43-48. https://doi.org/10.1053/j.semvascsurg.2019.02.001.
Dennis F. Bandyk 2018The diabetic foot: pathophysiology, evaluation, and treatmentSeminars in Vascular Surgery312-4434810.1053/j.semvascsurg.2019.02.001
Bimstein, E., D. Zangen, W. Abedrahim, and J. Katz. (2019). Type 1 diabetes mellitus (juvenile diabetes) - A review for the pediatric oral health provider. Journal of Clinical Pediatric Dentistry, 43(6): 417-23. https://doi.org/10.17796/1053-4625-43.6.10.
E. Bimstein D. Zangen W. Abedrahim J. Katz 2019Type 1 diabetes mellitus (juvenile diabetes) - A review for the pediatric oral health providerJournal of Clinical Pediatric Dentistry43641742310.17796/1053-4625-43.6.10
Burgess, Jamie L., W. Austin Wyant, Beatriz Abdo Abujamra, Robert S. Kirsner, and Ivan Jozic. (2021). Diabetic wound-healing science. Medicina, 57(10): 1072. https://doi.org/10.3390/medicina57101072.
Jamie L. Burgess W. Austin Wyant Beatriz Abdo Abujamra Robert S. Kirsner Ivan Jozic 2021Diabetic wound-healing scienceMedicina5710107210.3390/medicina57101072
Cacicedo González, R., C. Castañeda Robles, F. Cossío Gómez, A. Delgado Uría, B. Fernández Saíz, M. V. Gómez España, A. Gómez Fernández, et al. (2011). Manual de prevención y cuidados locales de heridas crónicas [Manual of prevention and local care of chronic wounds]. Santander, Cantabria, Spain: Servicio Cántabro de Salud.
R. Cacicedo González C. Castañeda Robles F. Cossío Gómez A. Delgado Uría B. Fernández Saíz M. V. Gómez España A. Gómez Fernández 2011Manual de prevención y cuidados locales de heridas crónicas [Manual of prevention and local care of chronic wounds]SantanderCantabria, SpainServicio Cántabro de Salud
Chand Mali, Suresh, Anita Dhaka, Sheetal Sharma, and Rohini Trivedi. (2023). Review on biogenic synthesis of copper nanoparticles and its potential applications. Inorganic Chemistry Communications, 149(March): 110448. https://doi.org/10.1016/j.inoche.2023.110448.
Suresh Chand Mali Anita Dhaka Sheetal Sharma Rohini Trivedi 032023Review on biogenic synthesis of copper nanoparticles and its potential applicationsInorganic Chemistry Communications149March11044810.1016/j.inoche.2023.110448
Chatterjee, Arijit Kumar, Ruchira Chakraborty, and Tarakdas Basu. (2014). Mechanism of antibacterial activity of copper nanoparticles. Nanotechnology, 25(13): 135101. https://doi.org/10.1088/0957-4484/25/13/135101.
Arijit Kumar Chatterjee Ruchira Chakraborty Tarakdas Basu 2014Mechanism of antibacterial activity of copper nanoparticlesNanotechnology251313510110.1088/0957-4484/25/13/135101
Chen, Dong, Steven B. Waters, Kathleen H. Holt, and Jeffrey E. Pessin. (1996). SOS phosphorylation and disassociation of the Grb2-SOS complex by the ERK and JNK signaling pathways. Journal of Biological Chemistry, 271(11): 6328-32. https://doi.org/10.1074/jbc.271.11.6328.
Dong Chen Steven B. Waters Kathleen H. Holt Jeffrey E. Pessin 1996SOS phosphorylation and disassociation of the Grb2-SOS complex by the ERK and JNK signaling pathwaysJournal of Biological Chemistry271116328633210.1074/jbc.271.11.6328
Christaki, Eirini, Markella Marcou, and Andreas Tofarides. (2020). Antimicrobial resistance in bacteria: mechanisms, evolution, and persistence. Journal of Molecular Evolution, 88(1): 2640. https://doi.org/10.1007/s00239-019-09914-3.
Eirini Christaki Markella Marcou Andreas Tofarides 2020Antimicrobial resistance in bacteria: mechanisms, evolution, and persistenceJournal of Molecular Evolution881264010.1007/s00239-019-09914-3
Christensen, Niels Juel. (1974). Plasma norepinephrine and epinephrine in untreated diabetics, during fasting and after insulin administration. Diabetes, 23(1): 1-8. https://doi.org/10.2337/diab.23.1.1.
Niels Juel Christensen 1974Plasma norepinephrine and epinephrine in untreated diabetics, during fasting and after insulin administrationDiabetes23118
Chupin, Maurice, Bernard Charbonnel, and Françoise Chupin. (1981). C-peptide blood levels in keto-acidosis and in hyperosmolar non-ketotic diabetic coma. Acta Diabetologica Latina, 18(2): 123-28. https://doi.org/10.1007/BF02098997.
Maurice Chupin Bernard Charbonnel Françoise Chupin 1981C-peptide blood levels in keto-acidosis and in hyperosmolar non-ketotic diabetic comaActa Diabetologica Latina18212312810.1007/BF02098997
Cline, Susan D. (2012). Mitochondrial DNA damage and its consequences for mitochondrial gene expression. Biochimica et Biophysica Acta (BBA) - Gene Regulatory Mechanisms, 1819(9-10): 979-91. https://doi.org/10.1016/j.bbagrm.2012.06.002.
Susan D. Cline 2012Mitochondrial DNA damage and its consequences for mitochondrial gene expressionBiochimica et Biophysica Acta (BBA) - Gene Regulatory Mechanisms18199-1097999110.1016/j.bbagrm.2012.06.002
Crisan, Michaela Corina, Mocan Teodora, and Mocan Lucian. (2021). Copper nanoparticles: synthesis and characterization, physiology, toxicity and antimicrobial applications. Applied Sciences, 12(1): 141. https://doi.org/10.3390/app12010141.
Michaela Corina Crisan Mocan Teodora Mocan Lucian 2021Copper nanoparticles: synthesis and characterization, physiology, toxicity and antimicrobial applicationsApplied Sciences12114110.3390/app12010141
Cutting, Keith F. (2003). Wound exudate: composition and functions. British Journal of Community Nursing, 8(Sup 3): S4-9. https://doi.org/10.12968/bjcn.2003.8.Sup3.11577.
Keith F. Cutting 2003Wound exudate: composition and functionsBritish Journal of Community Nursing83S4S910.12968/bjcn.2003.8.Sup3.11577
Deckers, Julie, Hamida Hammad, and Esther Hoste. (2018). Langerhans cells: sensing the environment in health and disease. Frontiers in Immunology, 9(February). https://doi.org/10.3389/fimmu.2018.00093.
Julie Deckers Hamida Hammad Esther Hoste 022018Langerhans cells: sensing the environment in health and diseaseFrontiers in Immunology9February10.3389/fimmu.2018.00093
DeFronzo, Ralph A. (2004). Pathogenesis of type 2 diabetes mellitus. Medical Clinics of North America, 88(4): 787-835. https://doi.org/10.1016/j.mcna.2004.04.013.
Ralph A. DeFronzo 2004Pathogenesis of type 2 diabetes mellitusMedical Clinics of North America88478783510.1016/j.mcna.2004.04.013
Ebina, Yousuke, Leland Ellis, Kurt Jarnagin, Marc Edery, Laszlo Graf, Eric Clauser, Jing-hsiung Ou, et al. (1985). The human insulin receptor CDNA: the structural basis for hormone-activated transmembrane signalling. Cell, 40(4): 747-58. https://doi.org/10.1016/0092-8674(85)90334-4.
Yousuke Ebina Leland Ellis Kurt Jarnagin Marc Edery Laszlo Graf Eric Clauser Jing-hsiung Ou 1985The human insulin receptor CDNA: the structural basis for hormone-activated transmembrane signallingCell40474775810.1016/0092-8674(85)90334-4
Forsberg, Jonathan Agner, Benjamin Kyle Potter, George Cierny, and Lawrence Webb. (2011). Diagnosis and management of chronic infection. American Academy of Orthopaedic Surgeon, 19(February): S8-19. https://doi.org/10.5435/00124635-201102001-00003.
Jonathan Agner Forsberg Benjamin Kyle Potter George Cierny Lawrence Webb 022011Diagnosis and management of chronic infectionAmerican Academy of Orthopaedic Surgeon19FebruaryS81910.5435/00124635-201102001-00003
Frydrych, Lynn M, Guowu Bian, David E O’Lone, Peter A Ward, and Matthew J Delano. (2018). Obesity and type 2 diabetes mellitus drive immune dysfunction, infection development, and sepsis mortality. Journal of Leukocyte Biology, 104 (3): 525-34. https://doi.org/10.1002/JLB.5VMR0118-021RR.
Lynn M Frydrych Guowu Bian O’Lone David E Peter A Ward Matthew J Delano 2018Obesity and type 2 diabetes mellitus drive immune dysfunction, infection development, and sepsis mortalityJournal of Leukocyte Biology104352553410.1002/JLB.5VMR0118-021RR
Frykberg, Robert G., and Jaminelli Banks. (2015). Challenges in the treatment of chronic wounds. Advances in Wound Care, 4(9): 560-82. https://doi.org/10.1089/wound.2015.0635.
Robert G. Frykberg Jaminelli Banks 2015Challenges in the treatment of chronic woundsAdvances in Wound Care4956058210.1089/wound.2015.0635
Galicia-García, Unai, Asier Benito-Vicente, Shifa Jebari, Asier Larrea-Sebal, Haziq Siddiqi, Kepa B. Uribe, Helena Ostolaza, and César Martín. (2020). Pathophysiology of type 2 diabetes mellitus. International Journal of Molecular Sciences, 21(17): 6275. https://doi.org/10.3390/ijms21176275.
Unai Galicia-García Asier Benito-Vicente Shifa Jebari Asier Larrea-Sebal Haziq Siddiqi Kepa B. Uribe Helena Ostolaza César Martín 2020Pathophysiology of type 2 diabetes mellitusInternational Journal of Molecular Sciences2117627510.3390/ijms21176275
Geerlings, Suzanne E., and Andy I. M. Hoepelman. (1999). Immune dysfunction in patients with diabetes mellitus (DM). FEMS Immunology & Medical Microbiology, 26(3-4): 259-65. https://doi.org/10.1111/j.1574-695X.1999.tb01397.x.
Suzanne E. Geerlings Andy I. M. Hoepelman 1999Immune dysfunction in patients with diabetes mellitus (DM)FEMS Immunology & Medical Microbiology263-425926510.1111/j.1574-695X.1999.tb01397.x
Goldberg, Stephanie R., and Robert F. Diegelmann. (2020). What makes wounds chronic. Surgical Clinics of North America, 100(4): 681-93. https://doi.org/10.1016/j.suc.2020.05.001.
Stephanie R. Goldberg Robert F. Diegelmann 2020What makes wounds chronicSurgical Clinics of North America100468169310.1016/j.suc.2020.05.001
Golden, Thea N., and Rebecca A. Simmons. (2021). Immune dysfunction in developmental programming of type 2 diabetes mellitus. Nature Reviews Endocrinology, 17(4): 235-45. https://doi.org/10.1038/s41574-020-00464-z.
Thea N. Golden Rebecca A. Simmons 2021Immune dysfunction in developmental programming of type 2 diabetes mellitusNature Reviews Endocrinology17423524510.1038/s41574-020-00464-z
Hassan, Esraa Mamdouh, Hisham Mushtaq, Esraa Elaraby Mahmoud, Sherley Chhibber, Shoaib Saleem, Ahmed Issa, Jain Nitesh, et al. (2022). Overlap of diabetic ketoacidosis and hyperosmolar hyperglycemic state. World Journal of Clinical Cases 10(32): 11702-11. https://doi.org/10.12998/wjcc.v10.i32.11702.
Esraa Mamdouh Hassan Hisham Mushtaq Esraa Elaraby Mahmoud Sherley Chhibber Shoaib Saleem Ahmed Issa Jain Nitesh 2022Overlap of diabetic ketoacidosis and hyperosmolar hyperglycemic stateWorld Journal of Clinical Cases1032117021171110.12998/wjcc.v10.i32.11702
Hayes, R. E., L. S. Mukadi, M. Votsmeier, and J. Gieshoff. (2004). Three-way catalytic converter modelling with detailed kinetics and washcoat diffusion. Topics in Catalysis, 30/31(July): 411-15. https://doi.org/10.1023/B:TOCA.0000029783.16199.f3.
R. E. Hayes L. S. Mukadi M. Votsmeier J. Gieshoff 072004Three-way catalytic converter modelling with detailed kinetics and washcoat diffusionTopics in Catalysis30/31July41141510.1023/B:TOCA.0000029783.16199.f3
Hiatt, William R., Ehrin J. Armstrong, Christopher J. Larson, and Eric P. Brass. (2015). Pathogenesis of the limb manifestations and exercise limitations in peripheral artery disease. Circulation Research, 116(9): 1527-39. https://doi.org/10.1161/CIRCRESAHA.116.303566.
William R. Hiatt Ehrin J. Armstrong Christopher J. Larson Eric P. Brass 2015Pathogenesis of the limb manifestations and exercise limitations in peripheral artery diseaseCirculation Research11691527153910.1161/CIRCRESAHA.116.303566
Hirano, Toshio. (2021). IL-6 in inflammation, autoimmunity and cancer. International Immunology, 33(3): 127-48. https://doi.org/10.1093/intimm/dxaa078.
Toshio Hirano 2021IL-6 in inflammation, autoimmunity and cancerInternational Immunology33312714810.1093/intimm/dxaa078
Hua, Camille, Tomas Urbina, Romain Bosc, Tom Parks, Shiranee Sriskandan, Nicolas de Prost, and Olivier Chosidow. (2023). Necrotising soft-tissue infections. The Lancet Infectious Diseases, 23(3): e81-94. https://doi.org/10.1016/S1473-3099(22)00583-7.
Camille Hua Tomas Urbina Romain Bosc Tom Parks Shiranee Sriskandan Nicolas de Prost Olivier Chosidow 2023Necrotising soft-tissue infectionsThe Lancet Infectious Diseases233e81e9410.1016/S1473-3099(22)00583-7
Jenks, Jeffrey D., Oliver A. Cornely, Sharon C.‐A. Chen, George R. Thompson, and Martin Hoenigl. (2020). Breakthrough invasive fungal infections: who is at risk? Mycoses, 63(10): 1021-32. https://doi.org/10.1111/myc.13148.
Jeffrey D. Jenks Oliver A. Cornely Sharon C.‐A. Chen George R. Thompson Martin Hoenigl 2020Breakthrough invasive fungal infections: who is at risk?Mycoses63101021103210.1111/myc.13148
Jiménez, Estanislao, Aida Hamdan-Partida, Francisco J. Padilla-Godínez, Daniela Arellano-Lara, Esteban Gómez-López, and Tessy M. López-Goerne. (2022). Spectroscopic analysis and microbicidal effect of Ag/TiO2-SiO2 bionanocatalysts. IEEE Transactions on NanoBioscience, 21(2): 246-55. https://doi.org/10.1109/TNB.2021.3122084.
Estanislao Jiménez Aida Hamdan-Partida Francisco J. Padilla-Godínez Daniela Arellano-Lara Esteban Gómez-López Tessy M. López-Goerne 2022Spectroscopic analysis and microbicidal effect of Ag/TiO2-SiO2 bionanocatalystsIEEE Transactions on NanoBioscience21224625510.1109/TNB.2021.3122084
Jones, Ruth Ellen, Deshka S. Foster, and Michael T. Longaker. (2018). Management of chronic wounds - 2018. JAMA, 320(14): 1481. https://doi.org/10.1001/jama.2018.12426.
Ruth Ellen Jones Deshka S. Foster Michael T. Longaker 2018Management of chronic wounds - 2018JAMA32014148110.1001/jama.2018.12426
Kahaly, George J., and Martin P. Hansen. (2016). Type 1 diabetes associated autoimmunity. Autoimmunity Reviews, 15(7): 644-48. https://doi.org/10.1016/j.autrev.2016.02.017.
George J. Kahaly Martin P. Hansen 2016Type 1 diabetes associated autoimmunityAutoimmunity Reviews15764464810.1016/j.autrev.2016.02.017
Kipnis, D. M. (1968). Insulin secretion in diabetes mellitus. Annals of Internal Medicine, 69(5): 891. https://doi.org/10.7326/0003-4819-69-5-891.
D. M. Kipnis 1968Insulin secretion in diabetes mellitusAnnals of Internal Medicine69589110.7326/0003-4819-69-5-891
Kitabchi, A. E., R. Young, H. Sacks, and L. Morris. 1979. Diabetic ketoacidosis: reappraisal of therapeutic approach. Annual Review of Medicine, 30(1): 339-57. https://doi.org/10.1146/annurev.me.30.020179.002011.
A. E. Kitabchi R. Young H. Sacks L. Morris 1979Diabetic ketoacidosis: reappraisal of therapeutic approachAnnual Review of Medicine30133935710.1146/annurev.me.30.020179.002011
Kitabchi, Abbas E. (1976). The efficacy of low-dose versus conventional therapy of insulin for treatment of diabetic ketoacidosis. Annals of Internal Medicine, 84(6): 633. https://doi.org/10.7326/0003-4819-84-6-633.
Abbas E. Kitabchi 1976The efficacy of low-dose versus conventional therapy of insulin for treatment of diabetic ketoacidosisAnnals of Internal Medicine84663310.7326/0003-4819-84-6-633
Kitabchi, Abbas E., and Ebenezer A. Nyenwe. (2006). Hyperglycemic crises in diabetes mellitus: diabetic ketoacidosis and hyperglycemic hyperosmolar state. Endocrinology and Metabolism Clinics of North America 35(4): 725-51. https://doi.org/10.1016/j.ecl.2006.09.006.
Abbas E. Kitabchi Ebenezer A. Nyenwe 2006Hyperglycemic crises in diabetes mellitus: diabetic ketoacidosis and hyperglycemic hyperosmolar stateEndocrinology and Metabolism Clinics of North America35472575110.1016/j.ecl.2006.09.006
Kitabchi, Abbas E., Guillermo E. Umpierrez, John M. Miles, and Joseph N. Fisher. (2009). Hyperglycemic crises in adult patients with diabetes. Diabetes Care, 32(7): 1335-43. https://doi.org/10.2337/dc09-9032.
Abbas E. Kitabchi Guillermo E. Umpierrez John M. Miles Joseph N. Fisher 2009Hyperglycemic crises in adult patients with diabetesDiabetes Care3271335134310.2337/dc09-9032
Kitabchi, Abbas E., Guillermo E. Umpierrez, Mary Beth Murphy, Eugene J. Barrett, Robert A. Kreisberg, John I. Malone, and Barry M. Wall. (2001). Management of hyperglycemic crises in patients with diabetes. Diabetes Care, 24(1): 131-53. https://doi.org/10.2337/diacare.24.1.131.
Abbas E. Kitabchi Guillermo E. Umpierrez Mary Beth Murphy Eugene J. Barrett Robert A. Kreisberg John I. Malone Barry M. Wall 2001Management of hyperglycemic crises in patients with diabetesDiabetes Care24113115310.2337/diacare.24.1.131
Kitabchi, Abbas E., and Barry M. Wall. (1995). Diabetic ketoacidosis. Medical Clinics of North America, 79(1): 9-37. https://doi.org/10.1016/S0025-7125(16)30082-7.
Abbas E. Kitabchi Barry M. Wall 1995Diabetic ketoacidosisMedical Clinics of North America79193710.1016/S0025-7125(16)30082-7
Knip, Mikael, and Heli Siljander. (2008). Autoimmune mechanisms in type 1 diabetes. Autoimmunity Reviews, 7(7): 550-57. https://doi.org/10.1016/j.autrev.2008.04.008.
Mikael Knip Heli Siljander 2008Autoimmune mechanisms in type 1 diabetesAutoimmunity Reviews7755055710.1016/j.autrev.2008.04.008
Lawrence, Michael C., Arif Jivan, Chunli Shao, Lingling Duan, Daryl Goad, Elma Zaganjor, Jihan Osborne, et al. (2008). The roles of MAPKs in disease. Cell Research, 18(4): 436-42. https://doi.org/10.1038/cr.2008.37.
Michael C. Lawrence Arif Jivan Chunli Shao Lingling Duan Daryl Goad Elma Zaganjor Jihan Osborne 2008The roles of MAPKs in diseaseCell Research18443644210.1038/cr.2008.37
Lepäntalo, Mauri, Leslie Fiengo, and Fausto Biancari. (2012). Peripheral arterial disease in diabetic patients with renal insufficiency: a review. Diabetes /Metabolism Research and Reviews, 28(February): 40-45. https://doi.org/10.1002/dmrr.2233.
Mauri Lepäntalo Leslie Fiengo Fausto Biancari 022012Peripheral arterial disease in diabetic patients with renal insufficiency: a reviewDiabetes /Metabolism Research and Reviews28February404510.1002/dmrr.2233
Li, Meirong, Qian Hou, Lingzhi Zhong, Yali Zhao, and Xiaobing Fu. (2021). Macrophage related chronic inflammation in non-healing wounds. Frontiers in Immunology, 12(June). https://doi.org/10.3389/fimmu.2021.681710.
Meirong Li Qian Hou Lingzhi Zhong Yali Zhao Xiaobing Fu 062021Macrophage related chronic inflammation in non-healing woundsFrontiers in Immunology12June10.3389/fimmu.2021.681710
Libby, Peter. (2021). The changing landscape of atherosclerosis. Nature, 592(7855): 524-33. https://doi.org/10.1038/s41586-021-03392-8.
Peter Libby 2021The changing landscape of atherosclerosisNature592785552453310.1038/s41586-021-03392-8
Liu, Ming, Jordan Wright, Huan Guo, Yi Xiong, and Peter Arvan. (2014). Proinsulin entry and transit through the endoplasmic reticulum in pancreatic beta cells. Vitamins and Hormons, 95: 35-62. https://doi.org/10.1016/B978-0-12-800174-5.00002-8.
Ming Liu Jordan Wright Huan Guo Yi Xiong Peter Arvan 2014Proinsulin entry and transit through the endoplasmic reticulum in pancreatic beta cellsVitamins and Hormons95356210.1016/B978-0-12-800174-5.00002-8
Liu, Wei, Yan Xu, and Vera I. Slaveykova. (2023). Oxidative stress induced by sub-lethal exposure to copper as a mediator in development of bacterial resistance to antibiotics. Science of the Total Environment, 860(February): 160516. https://doi.org/10.1016/j.scitotenv.2022.160516.
Wei Liu Yan Xu Vera I. Slaveykova 022023Oxidative stress induced by sub-lethal exposure to copper as a mediator in development of bacterial resistance to antibioticsScience of the Total Environment860February16051610.1016/j.scitotenv.2022.160516
López, T., J. L. Cuevas, L. Ilharco, P. Ramírez, F. Rodríguez Reinoso, and E. Rodríguez-Castelleón. (2018). XPS Characterization and E. Coli DNA degradation using functionalized Cu/TiO2 nanobiocatalysts. Molecular Catalysis, 449: 52-71. https://doi.org/10.1016/j.mcat.2018.02.010.
T. López J. L. Cuevas L. Ilharco P. Ramírez F. Rodríguez Reinoso E. Rodríguez-Castelleón 2018XPS Characterization and E. Coli DNA degradation using functionalized Cu/TiO2 nanobiocatalystsMolecular Catalysis449527110.1016/j.mcat.2018.02.010
López, T., G. Jardón, G. Gómez, A. Gracía, A. Hamdan, J. L. Cuevas, P. Quintana, and O. Novaro. (2015). Ag/TiO2-SiO2 sol-gel nanoparticles to use in hospital-acquired infections (HAI). Journal of Materials Science and Technology, 4(6): 1000196. https://doi.org/10.4172/2169-0022.1000196.
T. López G. Jardón G. Gómez A. Gracía A. Hamdan J. L. Cuevas P. Quintana O. Novaro 2015Ag/TiO2-SiO2 sol-gel nanoparticles to use in hospital-acquired infections (HAI)Journal of Materials Science and Technology46100019610.4172/2169-0022.1000196
López-Goerne, Tessy M., Francisco J. Padilla-Godínez, Mariana Castellanos, and Luis A. Pérez-Dávalos. (2022). Catalytic nanomedicine: a brief review of bionanocatalysts. Nanomedicine, 17(16): 1131-56. https://doi.org/10.2217/nnm-2022-0027.
Tessy M. López-Goerne Francisco J. Padilla-Godínez Mariana Castellanos Luis A. Pérez-Dávalos 2022Catalytic nanomedicine: a brief review of bionanocatalystsNanomedicine17161131115610.2217/nnm-2022-0027
López-Goerne, Tessy, Paola Ramírez, Alba Arévalo, Mireya Huantes, and Francisco J. Padilla-Godínez. (2022). Catalytic nanomedicine - A new approach and solution for chronic ulcers: case series. The International Journal of Lower Extremity Wounds, August, 153473462211190. https://doi.org/10.1177/15347346221119006.
Tessy López-Goerne Paola Ramírez Alba Arévalo Mireya Huantes Francisco J. Padilla-Godínez 082022Catalytic nanomedicine - A new approach and solution for chronic ulcers: case seriesThe International Journal of Lower Extremity WoundsAugust15347346221119010.1177/15347346221119006
Luo, Han, Zhen Wang, Fang Qi, and Dali Wang. (2022). Applications of human amniotic fluid stem cells in wound healing. Chinese Medical Journal, 135(19): 2272-81. https://doi.org/10.1097/CM9.0000000000002076.
Han Luo Zhen Wang Fang Qi Dali Wang 2022Applications of human amniotic fluid stem cells in wound healingChinese Medical Journal135192272228110.1097/CM9.0000000000002076
Mancusi, Costantino, Raffaele Izzo, Giuseppe di Gioia, Maria Angela Losi, Emanuele Barbato, and Carmine Morisco. (2020). Insulin resistance the hinge between hypertension and type 2 diabetes. High Blood Pressure & Cardiovascular Prevention, 27(6): 515-26. https://doi.org/10.1007/s40292-020-00408-8.
Costantino Mancusi Raffaele Izzo Giuseppe di Gioia Maria Angela Losi Emanuele Barbato Carmine Morisco 2020Insulin resistance the hinge between hypertension and type 2 diabetesHigh Blood Pressure & Cardiovascular Prevention27651552610.1007/s40292-020-00408-8
Meyts, Pierre de. (2004). Insulin and its receptor: structure, function and evolution. BioEssays, 26(12): 1351-62. https://doi.org/10.1002/bies.20151.
Pierre de Meyts 2004Insulin and its receptor: structure, function and evolutionBioEssays26121351136210.1002/bies.20151
Moini, Jahangir. (2019). Pathophysiology of diabetes. Epidemiology of Diabetes, 25-43. Elsevier. https://doi.org/10.1016/B978-0-12-816864-6.00003-1.
Jahangir Moini 2019Pathophysiology of diabetesEpidemiology of Diabetes2543Elsevier10.1016/B978-0-12-816864-6.00003-1
Molecular Basis of Insulin Action. (2007). Drug News & Perspectives, 20(8): 527. https://doi.org/10.1358/dnp.2007.20.8.1157615.
Molecular Basis of Insulin Action2007Drug News & Perspectives20852710.1358/dnp.2007.20.8.1157615
Morton, Laurel M., and Tania J. Phillips. (2016). Wound healing and treating wounds. Journal of the American Academy of Dermatology, 74(4): 589-605. https://doi.org/10.1016/j.jaad.2015.08.068.
Laurel M. Morton Tania J. Phillips 2016Wound healing and treating woundsJournal of the American Academy of Dermatology74458960510.1016/j.jaad.2015.08.068
Müller, Walter A., Gerald R. Faloona, and Roger H. Unger. (1973). Hyperglucagonemia in diabetic ketoacidosis. The American Journal of Medicine, 54(1): 52-57. https://doi.org/10.1016/0002-9343(73)90083-1.
Walter A. Müller Gerald R. Faloona Roger H. Unger 1973Hyperglucagonemia in diabetic ketoacidosisThe American Journal of Medicine541525710.1016/0002-9343(73)90083-1
Muoio, Deborah M., and Christopher B. Newgard. (2008). Molecular and metabolic mechanisms of insulin resistance and β-cell failure in type 2 diabetes. Nature Reviews Molecular Cell Biology, 9(3): 193-205. https://doi.org/10.1038/nrm2327.
Deborah M. Muoio Christopher B. Newgard 2008Molecular and metabolic mechanisms of insulin resistance and β-cell failure in type 2 diabetesNature Reviews Molecular Cell Biology9319320510.1038/nrm2327
Omar, Amin, J. Wright, Gregory Schultz, Robert Burrell, and Patricia Nadworny. (2017). Microbial biofilms and chronic wounds. Microorganisms, 5(1): 9. https://doi.org/10.3390/microorganisms5010009.
Amin Omar J. Wright Gregory Schultz Robert Burrell Patricia Nadworny 2017Microbial biofilms and chronic woundsMicroorganisms51910.3390/microorganisms5010009
Petersen, Max C., and Gerald I. Shulman. (2018). Mechanisms of insulin action and insulin resistance. Physiological Reviews, 98(4): 2133-2223. https://doi.org/10.1152/physrev.00063.2017.
Max C. Petersen Gerald I. Shulman 2018Mechanisms of insulin action and insulin resistancePhysiological Reviews9842133222310.1152/physrev.00063.2017
Petersmann, Astrid, Dirk Müller-Wieland, Ulrich A. Müller, Rüdiger Landgraf, Matthias Nauck, Guido Freckmann, Lutz Heinemann, and Erwin Schleicher. (2019). Definition, classification and diagnosis of diabetes mellitus. Experimental and Clinical Endocrinology & Diabetes, 127(S 01): S1-7. https://doi.org/10.1055/a-1018-9078.
Astrid Petersmann Dirk Müller-Wieland Ulrich A. Müller Rüdiger Landgraf Matthias Nauck Guido Freckmann Lutz Heinemann Erwin Schleicher 2019Definition, classification and diagnosis of diabetes mellitusExperimental and Clinical Endocrinology & Diabetes127S 01S1S710.1055/a-1018-9078
Peterson, L. R. (2005). Squeezing the antibiotic balloon: the impact of antimicrobial classes on emerging resistance. Clinical Microbiology and Infection, 11: 4-16. https://doi.org/10.1111/j.1469-0691.2005.01238.x.
L. R. Peterson 2005Squeezing the antibiotic balloon: the impact of antimicrobial classes on emerging resistanceClinical Microbiology and Infection1141610.1111/j.1469-0691.2005.01238.x
Peulen, Thomas-Otavio, and Kevin J. Wilkinson. (2011). Diffusion of nanoparticles in a biofilm. Environmental Science & Technology, 45(8): 3367-73. https://doi.org/10.1021/es103450g.
Thomas-Otavio Peulen Kevin J. Wilkinson 2011Diffusion of nanoparticles in a biofilmEnvironmental Science & Technology4583367337310.1021/es103450g
Ramyadevi, Jeyaraman, Kadarkaraithangam Jeyasubramanian, Arumugam Marikani, Govindasamy Rajakumar, and Abdul Abdul Rahuman. (2012). Synthesis and antimicrobial activity of copper nanoparticles. Materials Letters, 71(March): 114-16. https://doi.org/10.1016/j.matlet.2011.12.055.
Jeyaraman Ramyadevi Kadarkaraithangam Jeyasubramanian Arumugam Marikani Govindasamy Rajakumar Abdul Abdul Rahuman 2012Synthesis and antimicrobial activity of copper nanoparticlesMaterials Letters71March11411610.1016/j.matlet.2011.12.055
Ravichandran, Kodi S. (2001). Signaling via Shc family adapter proteins. Oncogene, 20(44): 6322-30. https://doi.org/10.1038/sj.onc.1204776.
Kodi S. Ravichandran 2001Signaling via Shc family adapter proteinsOncogene20446322633010.1038/sj.onc.1204776
Saltiel, Alan R., and C. Ronald Kahn. (2001a). Insulin signalling and the regulation of glucose and lipid metabolism. Nature, 414(6865): 799-806. https://doi.org/10.1038/414799a.
Alan R. Saltiel C. Ronald Kahn 2001Insulin signalling and the regulation of glucose and lipid metabolismNature414686579980610.1038/414799a
Saltiel, Alan R., and C. Ronald Kahn (2001b). Insulin signalling and the regulation of glucose and lipid metabolism. Nature, 414(6865): 799-806. https://doi.org/10.1038/414799a.
Alan R. Saltiel C. Ronald Kahn 2001Insulin signalling and the regulation of glucose and lipid metabolismNature414686579980610.1038/414799a
Stoitzner, Patrizia. (2010). The langerhans cell controversy: are they immunostimulatory or immunoregulatory cells of the skin immune system? Immunology & Cell Biology, 88(4): 348-50. https://doi.org/10.1038/icb.2010.46.
Patrizia Stoitzner 2010The langerhans cell controversy: are they immunostimulatory or immunoregulatory cells of the skin immune system?Immunology & Cell Biology88434835010.1038/icb.2010.46
Tokarz, Victoria L., Patrick E. MacDonald, and Amira Klip. (2018). The cell biology of systemic insulin function. Journal of Cell Biology, 217(7): 2273-89. https://doi.org/10.1083/jcb.201802095.
Victoria L. Tokarz Patrick E. MacDonald Amira Klip 2018The cell biology of systemic insulin functionJournal of Cell Biology21772273228910.1083/jcb.201802095
Versey, Zoya, Waleska Stephanie da Cruz Nizer, Emily Russell, Sandra Zigic, Katrina G. DeZeeuw, Jonah E. Marek, Joerg Overhage, and Edana Cassol. (2021). Biofilm-Innate immune interface: contribution to chronic wound formation. Frontiers in Immunology, 12(April). https://doi.org/10.3389/fimmu.2021.648554.
Zoya Versey Waleska Stephanie da Cruz Nizer Emily Russell Sandra Zigic Katrina G. DeZeeuw Jonah E. Marek Joerg Overhage Edana Cassol 042021Biofilm-Innate immune interface: contribution to chronic wound formationFrontiers in Immunology12April10.3389/fimmu.2021.648554
Vincent, Andrea M., Lorena Perrone, Kelli A. Sullivan, Carey Backus, Ann Marie Sastry, Christian Lastoskie, and Eva L. Feldman. (2007). Receptor for advanced glycation end products activation injures primary sensory neurons via oxidative stress. Endocrinology, 148(2): 548-58. https://doi.org/10.1210/en.2006-0073.
Andrea M. Vincent Lorena Perrone Kelli A. Sullivan Carey Backus Ann Marie Sastry Christian Lastoskie Eva L. Feldman 2007Receptor for advanced glycation end products activation injures primary sensory neurons via oxidative stressEndocrinology148254855810.1210/en.2006-0073
Virally, M., J.-F. Blicklé, J. Girard, S. Halimi, D. Simon, and P.-J. Guillausseau. (2007). Type 2 diabetes mellitus: epidemiology, pathophysiology, unmet needs and therapeutical perspectives. Diabetes & Metabolism, 33(4): 231-44. https://doi.org/10.1016/j.diabet.2007.07.001.
M. Virally J.-F. Blicklé J. Girard S. Halimi D. Simon P.-J. Guillausseau 2007Type 2 diabetes mellitus: epidemiology, pathophysiology, unmet needs and therapeutical perspectivesDiabetes & Metabolism33423124410.1016/j.diabet.2007.07.001
Wada, Ryuichi, and Soroku Yagihashi. (2005). Role of advanced glycation end products and their receptors in development of diabetic neuropathy. Annals of the New York Academy of Sciences, 1043(1): 598-604. https://doi.org/10.1196/annals.1338.067.
Ryuichi Wada Soroku Yagihashi 2005Role of advanced glycation end products and their receptors in development of diabetic neuropathyAnnals of the New York Academy of Sciences1043159860410.1196/annals.1338.067
Wang, Xiao‐Xiong, Qi Liu, Jin‐Xia Sui, Seeram Ramakrishna, Miao Yu, Yu Zhou, Xing‐Yu Jiang, and Yun‐Ze Long. (2019). Recent advances in hemostasis at the nanoscale. Advanced Healthcare Materials, 8(23): 1900823. https://doi.org/10.1002/adhm.201900823.
Xiao‐Xiong Wang Qi Liu Jin‐Xia Sui Seeram Ramakrishna Miao Yu Yu Zhou Xing‐Yu Jiang Yun‐Ze Long 2019Recent advances in hemostasis at the nanoscaleAdvanced Healthcare Materials823190082310.1002/adhm.201900823
Weisel, John W., and Rustem I. Litvinov. (2017). Fibrin formation, structure and properties. In Parry, D., Squire, J. (eds) Fibrous proteins: structures and mechanisms. Subcellular biochemistry, vol. 82. Springer, 405-56. https://doi.org/10.1007/978-3-319-49674-0_13.
John W. Weisel Rustem I. Litvinov 2017Fibrin formation, structure and properties D. Parry J. Squire Fibrous proteins: structures and mechanisms. Subcellular biochemistry82Springer40545610.1007/978-3-319-49674-0_13
Williams, H. C., R. P. Dellavalle, and S. Garner. (2012). Acne vulgaris. The Lancet, 379(9813): 361-72. https://doi.org/10.1016/S0140-6736(11)60321-8.
H. C. Williams R. P. Dellavalle S. Garner 2012Acne vulgarisThe Lancet379981336137210.1016/S0140-6736(11)60321-8
Wolcott, R. D., S. B. Cox, and S. E. Dowd. (2010). Healing and healing rates of chronic wounds in the age of molecular pathogen diagnostics. Journal of Wound Care, 19(7): 272-78, 280-81.
R. D. Wolcott S. B. Cox S. E. Dowd 2010Healing and healing rates of chronic wounds in the age of molecular pathogen diagnosticsJournal of Wound Care197272 280-81272-78, 280-81
Wolcott, R. D., and D. D. Rhoads. (2008). A study of biofilm-based wound management in subjects with critical limb ischaemia. Journal of Wound Care, 17(4): 145-48, 150-52, 154-55. https://doi.org/10.12968/jowc.2008.17.4.28835.
R. D. Wolcott D. D. Rhoads 2008A study of biofilm-based wound management in subjects with critical limb ischaemiaJournal of Wound Care174145 154-55145-48, 150-52, 154-5510.12968/jowc.2008.17.4.28835
Wolcott, R. D., D. D. Rhoads, and S. E. Dowd. (2008). Biofilms and chronic wound inflammation. Journal of Wound Care, 17(8): 333-41. https://doi.org/10.12968/jowc.2008.17.8.30796.
R. D. Wolcott D. D. Rhoads S. E. Dowd 2008Biofilms and chronic wound inflammationJournal of Wound Care17833334110.12968/jowc.2008.17.8.30796
Wu, Yuan-Kun, Nai-Chen Cheng, and Chao-Min Cheng. (2019). Biofilms in chronic wounds: pathogenesis and diagnosis. Trends in Biotechnology, 37(5): 505-17. https://doi.org/10.1016/j.tibtech.2018.10.011.
Yuan-Kun Wu Nai-Chen Cheng Chao-Min Cheng 2019Biofilms in chronic wounds: pathogenesis and diagnosisTrends in Biotechnology37550551710.1016/j.tibtech.2018.10.011
Yakes, F. Michael, and Bennett van Houten. (1997). Mitochondrial DNA damage is more extensive and persists longer than nuclear DNA damage in human cells following oxidative stress. Proceedings of the National Academy of Sciences, 94(2): 514-19. https://doi.org/10.1073/pnas.94.2.514.
F. Michael Yakes Bennett van Houten 1997Mitochondrial DNA damage is more extensive and persists longer than nuclear DNA damage in human cells following oxidative stressProceedings of the National Academy of Sciences94251451910.1073/pnas.94.2.514
Yamakawa, Sho, and Kenji Hayashida. (2019). Advances in surgical applications of growth factors for wound healing. Burns & Trauma, 7(December). https://doi.org/10.1186/s41038-019-0148-1.
Sho Yamakawa Kenji Hayashida 122019Advances in surgical applications of growth factors for wound healingBurns & Trauma7December10.1186/s41038-019-0148-1
Yin, Wen, Yiting Wang, Lu Liu, and Jin He. (2019). Biofilms: the microbial ‘protective clothing’ in extreme environments. International Journal of Molecular Sciences, 20(14): 3423. https://doi.org/10.3390/ijms20143423.
Wen Yin Yiting Wang Lu Liu Jin He 2019Biofilms: the microbial ‘protective clothing’ in extreme environmentsInternational Journal of Molecular Sciences2014342310.3390/ijms20143423
Youn, Young Jin, and Juyong Lee. (2019). Chronic venous insufficiency and varicose veins of the lower extremities. The Korean Journal of Internal Medicine, 34(2): 269-83. https://doi.org/10.3904/kjim.2018.230.
Young Jin Youn Juyong Lee 2019Chronic venous insufficiency and varicose veins of the lower extremitiesThe Korean Journal of Internal Medicine34226928310.3904/kjim.2018.230
Youngren, J. F. (2007). Regulation of insulin receptor function. Cell ular and Molecular Life Sciences, 64(7-8): 873-91. https://doi.org/10.1007/s00018-007-6359-9.
J. F. Youngren 2007Regulation of insulin receptor functionCell ular and Molecular Life Sciences647-887389110.1007/s00018-007-6359-9
Zick, Yehiel. (2005). Ser/Thr phosphorylation of IRS proteins: a molecular basis for insulin resistance. Science’s STKE, 2005(268). https://doi.org/10.1126/stke.2682005pe4.
Yehiel Zick 2005Ser/Thr phosphorylation of IRS proteins: a molecular basis for insulin resistanceScience’s STKE200526810.1126/stke.2682005pe4
Notes
[1] This work was supported by the Ministry of Health of Mexico City (grant number 101/100/014/13). The authors would like to acknowledge the expertise and technical help received from Alejandro Javier Velázquez-Muñoz, M.D., and Lois Regalado, M.D. Special thanks for the support of Nanomed Laboratorios, S.A. de C.V.